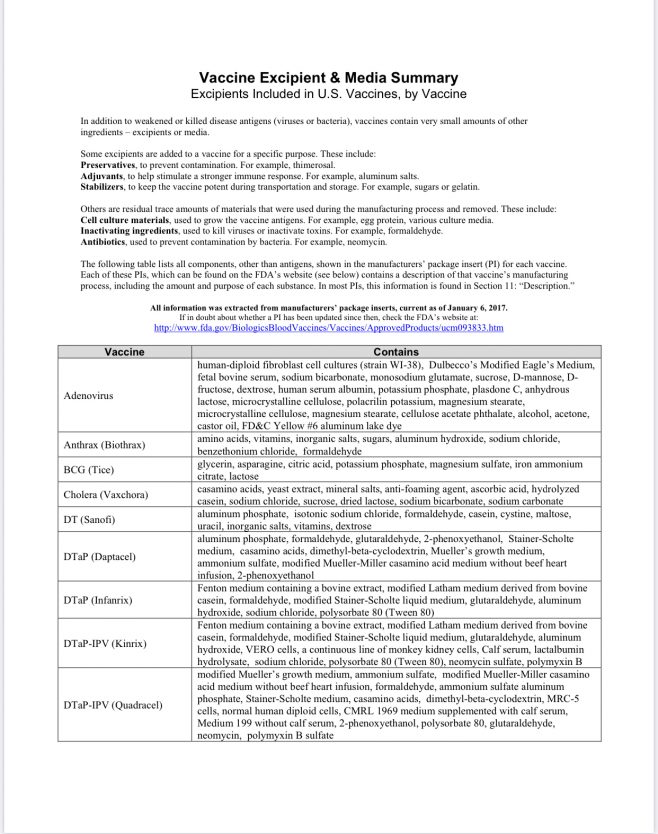
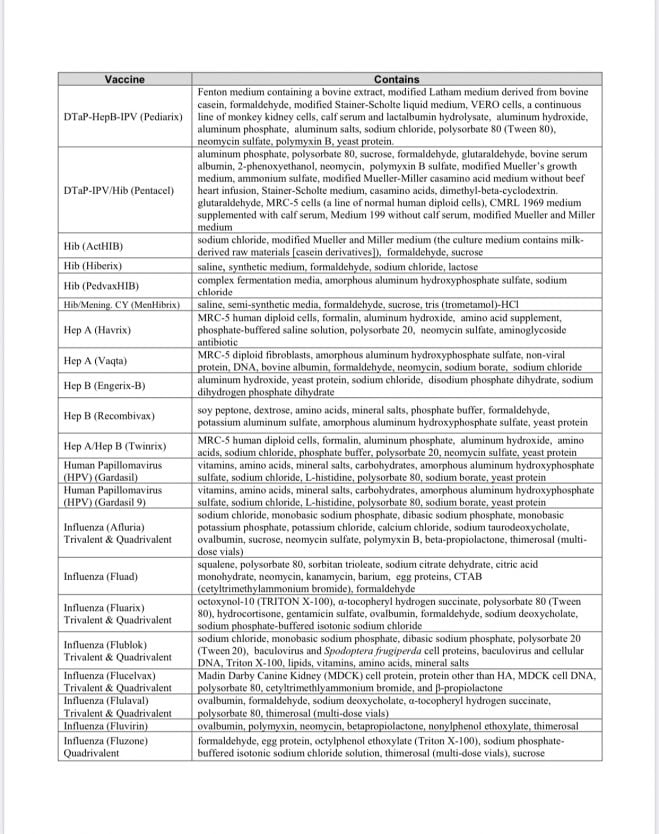
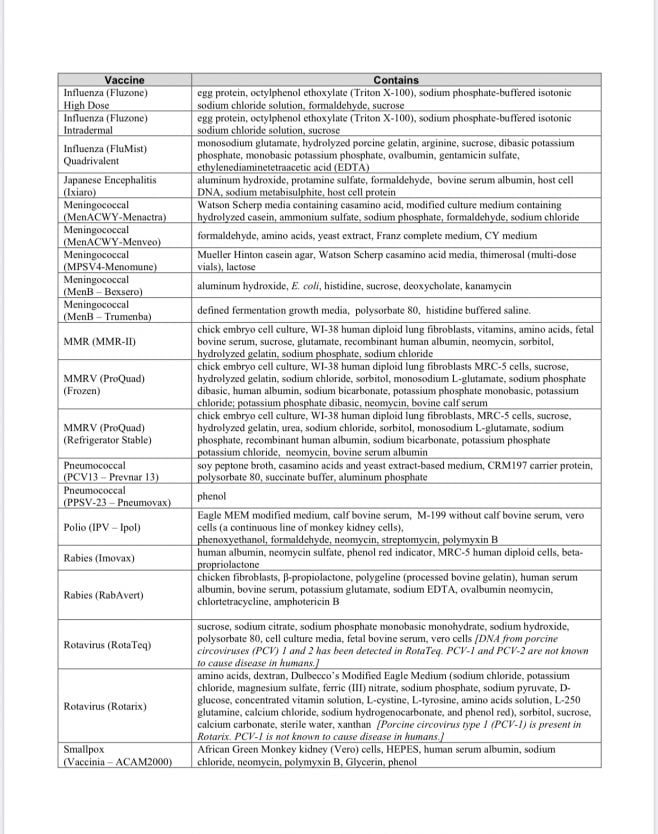
Vaccine Excipients and Media Summary
Avid product label-readers might be shocked by the information on this 2017 document posted on the CDC’s own website:
Recently this document was revised to separate out the vaccine excipents from the vaccine media used in production of vaccines; meanwhile, the vaccines continue to contain components of both excipients and media, presumably. [Refer to the document for a description of “excipients” and “media.” In general, they are the “other ingredients” of vaccines in addition to the “active” components.] The document states that the list is derived from vaccine manufacturers’ package inserts [which doctors and nurses typically don’t access in a clinical setting; you can read them online at the FDA website].
Note the inclusion of various species of animal tissue, including human fetal tissue derived from elective abortions. Typically, vaccine antigens* are grown on living cells of various types shown on this list. Unfortunately, the vaccine manufacturing process leaves behind these residues–they are not purified out of the vaccine, as people might anticipate.
*Antigen: part of a vaccine that is supposed to stimulate the vaccine recipient’s immune system to develop antibodies to fight off a subsequent exposure to a similar pathogen
Could injecting these vaccine contaminants into humans potentially contribute to confusion of the immune system, leading to autoimmunity? This question deserves to be answered for the public, especially considering the rise in autoimmune disorders during the vaccine era.
It may be of value to share this document with your healthcare providers for a discussion on the safety of injecting these ingredients, bypassing the body’s natural barriers against pathogens. Some vaccines, such as Hepatitis B, are given on Day #1 of life, to babies with immature immune systems. To date, no studies have been conducted on the cumulative effects of the entire CDC-recommended childhood or adult vaccination schedules.
The information above comes strictly from the manufacturers’ own declarations of their products’ ingredients. An Italian group, Corvelva, recently has been reporting on their laboratory discoveries that the labels of the particular vaccine vials they have analyzed were not congruent with what was stated on the product labels/packaging literature, calling into question vaccine manufacturers’ quality control.
It behooves parents to do their due diligence to understand what it is that is being injected into their children and to learn about the potential risks to health. The CDC-recommended schedule has expanded considerably since the introduction of the polio vaccine in the 1950s – a topic for a future discussion.